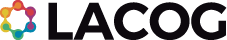A randomized phase III trial of palbociclib with adjuvant endocrine therapy versus endocrine therapy alone for hormone receptor positive (HR+)/ human epidermal growth factor receptor 2 (HER2)-negative early breast Cancer
Type of Study: Clinical Trial
Sponsor / Support: ABCSG, ALLIANCE
Primary Objectives: To compare invasive disease-free survival (iDFS) for the combination of at least 5 years of endocrine therapy plus 2 years of palbociclib treatment versus at least 5 years endocrine therapy alone in patients with histologically confirmed HR-positive/HER2-negative early breast cancer (EBC).
Design: This is a prospective, international, multicenter, randomized, open label Phase III study evaluating the addition of 2 years of palbociclib to at least 5 years of standard adjuvant endocrine therapy for patients with HR+/ HER2-negative EBC.
Sample Size: 4.600 patients
Principal Investigator: Gustavo Werutsky (Steering Committee – LACOG)
Countries LATAM: Mexico
Ongoing studies
Breast
GBECAM 0115 – AMAZONA III
LACOG 0115 – LORELEI
LACOG 0221 – BRAVE
LACOG 0413 – Male Breast Cancer
LACOG 0419 – NEOSAMBA
LACOG 0615 – LATINABreast
LACOG 0715 – PALLAS
LACOG 0721 – Cherry Pick
LACOG 2118 – ALEXANDRA/IMpassion 030
Gastrointestinal
LACOG/GTG 0119 – NET
LACOG 0222 – GASPAR
LACOG 0421 – ACTION HIV
LACOG/GTG 1318 – Anal Registry
Genitourinary
LACOG 0121 – PET-PSMA
LACOG 0217 – IRONMAN
LACOG 0218 – Hercules
LACOG 0519 – PEACE III
LACOG 0522 – DORA
LACOG 0620 – ExBAT
LACOG 1120 – RENAL REGISTRY
LACOG 1818 – Prostate Cancer Registry
LACOG 2018 – Foundation Penile
Gynecological
EVA/LACOG 0123 | GOG-3043 – ROCC Trial
LACOG 0223 / GOG-3073 – ROSELLA
LACOG 0521 – MADONNA
LACOG 0623 – eVOLVE-Cervical
LACOG 0820 – EVITA LATAM
LACOG 0920 – Senticol III
LACOG 1018 – PALBO in Ovarian Cancer
LACOG 1220 – CONOR
Head and Neck
LACOG 0319
LACOG 0720 – SMART-KEY
Lung
LACOG 0116 – LATINO LUNG
LACOG 0118 – RELANCE
LACOG 0120 – EAP durva lung cancer
LACOG 0322 – EDUR-BRA
LACOG 0821
LACOG 1918
LACOG 2218 – PACIFIC BRAZIL
Radiation
LACOG 0122 – RADIANT
Closed studies
Breast
LACOG 0111 – MGH
LACOG 0312 – METASTATIC BC
LACOG 0414
LACOG 0801 – GLICO
Genitourinary
LACOG 0415 – APA in Prostate Cancer
LACOG 0515 – Testicular Registry
LACOG 1518 – Bladder Cancer Registry
Gynecological
LACOG 0215 – EVITA
Head and Neck
LACOG 0318 – Induction
Lung
LACOG 0211 – ALK
LACOG 0417 – CNS MTX
LACOG 0618
Parsimony
Neuro
LACOG 0619
Other studies
LACOG 0213 – AUGMENT
LACOG 0214 – FINEP